Protaktiniy
91
Pa
Guruh
Nomaʼlum
Davr
7
Blok
f
Protonlar
Elektronlar
Neytronlar
91
91
140
Umumiy xususiyatlar
Atom raqami
91
Atom massasi
231,03588
Mass raqami
231
Turkum
Aktinoidlar
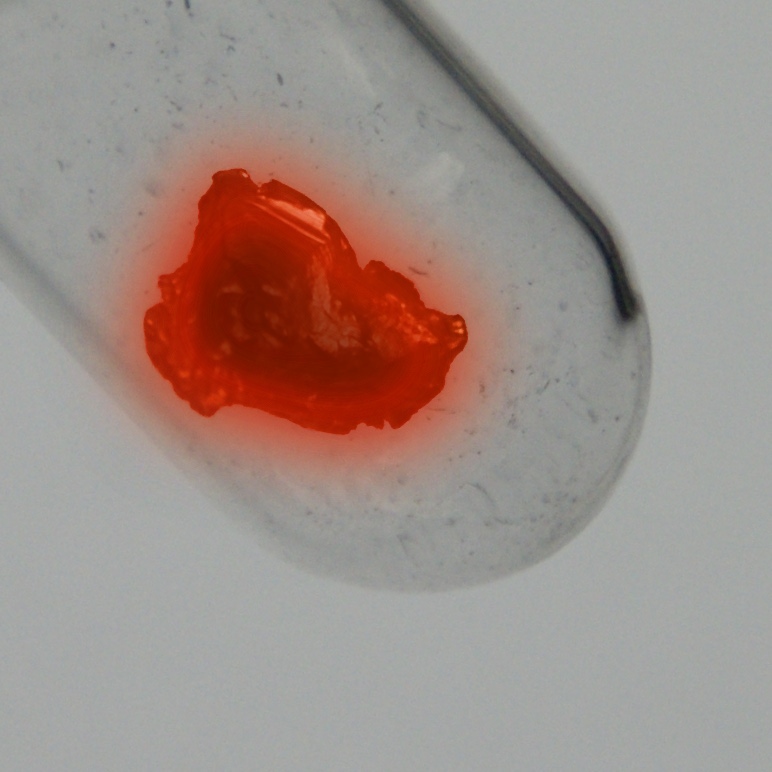
Ranggi
Kumush
Radioaktivlik
Ha
From the Greek protos meaning first
Kristall tuzilma
Markazli tetragonal
Tarix
In 1900, William Crookes isolated protactinium as an intensely radioactive material from uranium
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Qobiqdagi elektronlar soni
2, 8, 18, 32, 20, 9, 2
Elektron konfiguratsiyasi
[Rn] 5f2 6d1 7s2
Protactinium is one of the rarest and most expensive naturally occurring elements
Jismoniy xususiyatlar
Faza
Qattiq modda
Zichlik
15,37 g/cm3
Erish harorati
1841,15 K | 1568 °C | 2854,4 °F
Qaynash harorati
4300,15 K | 4027 °C | 7280,6 °F
Eritish issiqligi
15 kJ/mol
Bugʻlanish issiqligi
470 kJ/mol
Solishtirma issiqlik sigʻimi
- J/g·K
Yer po‘stidagi miqdori
9,9×10-13%
Olamdagi miqdori
Nomaʼlum
CAS raqami
7440-13-3
PubChem CID raqami
Nomaʼlum
Atom xususiyatlari
Atom radiusi
163 pm
Kovalentlik radiusi
200 pm
Elektrmanfiylik
1,5 (Poling boʻyicha)
Ionlashish potensiali
5,89 eV
Molyar hajm
15,0 cm3/mol
Issiqlik oʻtkazuvchanlik
0,47 W/cm·K
Oksidlanish darajasi
3, 4, 5
Qo‘llanilish sohalari
Owing to its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside of scientific research.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
Protactinium is toxic and highly radioactive
Izotoplar
Barqaror izotoplar
-Beqaror izotoplar
212Pa, 213Pa, 214Pa, 215Pa, 216Pa, 217Pa, 218Pa, 219Pa, 220Pa, 221Pa, 222Pa, 223Pa, 224Pa, 225Pa, 226Pa, 227Pa, 228Pa, 229Pa, 230Pa, 231Pa, 232Pa, 233Pa, 234Pa, 235Pa, 236Pa, 237Pa, 238Pa, 239Pa, 240Pa