Poloniy
84
Po
Guruh
16
Davr
6
Blok
p
Protonlar
Elektronlar
Neytronlar
84
84
126
Umumiy xususiyatlar
Atom raqami
84
Atom massasi
[210]
Mass raqami
210
Turkum
Yarim metallar
Ranggi
Kumush
Radioaktivlik
Ha
Named after Poland, native country of Madam Curie
Kristall tuzilma
Oddiy kubik
Tarix
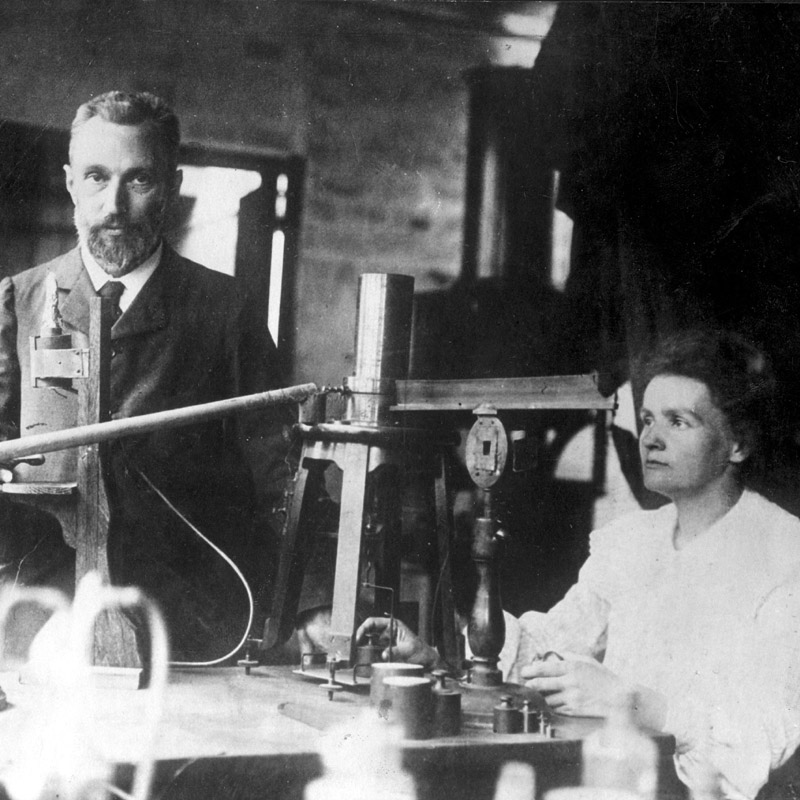
Polonium was discovered by Marie and Pierre Curie in 1898 in Paris.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
Qobiqdagi elektronlar soni
2, 8, 18, 32, 18, 6
Elektron konfiguratsiyasi
[Xe] 4f14 5d10 6s2 6p4
Polonium is obtained by irradiating bismuth with high-energy neutrons or protons
Jismoniy xususiyatlar
Faza
Qattiq modda
Zichlik
9,196 g/cm3
Erish harorati
527,15 K | 254 °C | 489,2 °F
Qaynash harorati
1235,15 K | 962 °C | 1763,6 °F
Eritish issiqligi
13 kJ/mol
Bugʻlanish issiqligi
100 kJ/mol
Solishtirma issiqlik sigʻimi
- J/g·K
Yer po‘stidagi miqdori
Nomaʼlum
Olamdagi miqdori
Nomaʼlum
CAS raqami
7440-08-6
PubChem CID raqami
Nomaʼlum
Atom xususiyatlari
Atom radiusi
168 pm
Kovalentlik radiusi
140 pm
Elektrmanfiylik
2,00 (Poling boʻyicha)
Ionlashish potensiali
8,417 eV
Molyar hajm
22,23 cm3/mol
Issiqlik oʻtkazuvchanlik
0,2 W/cm·K
Oksidlanish darajasi
-2, 2, 4, 6
Qo‘llanilish sohalari
Polonium is used to eliminate static electricity produced during processes such as rolling paper, wire and sheet metal.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium is highly dangerous and radioactive
Izotoplar
Barqaror izotoplar
-Beqaror izotoplar
188Po, 189Po, 190Po, 191Po, 192Po, 193Po, 194Po, 195Po, 196Po, 197Po, 198Po, 199Po, 200Po, 201Po, 202Po, 203Po, 204Po, 205Po, 206Po, 207Po, 208Po, 209Po, 210Po, 211Po, 212Po, 213Po, 214Po, 215Po, 216Po, 217Po, 218Po, 219Po, 220Po